New FDA Warnings for Prenatal Screening Tests and False Positives
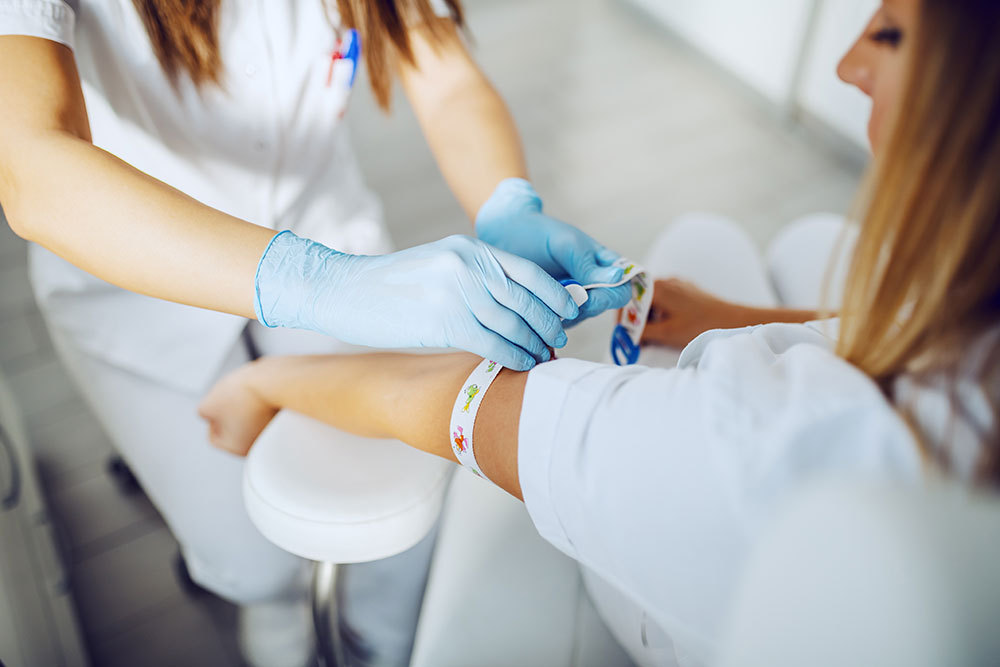
What expectant women need to know when interpreting results and making decisions for their pregnancies.
The U.S. Food and Drug Administration (FDA) is sounding the alarm on the risk of faulty results associated with non-invasive prenatal screening (NIPS) tests. This genetic testing is performed in the first trimester and is offered as a way for parents to gain “peace of mind” by ensuring there are no genetic abnormalities present in the developing fetus. However, a report from The New York Times indicates these tests are largely inaccurate (up to 85% of the time!) when screening for very rare genetic conditions, and pose a threat to patients using results to make choices about their health and their pregnancy.
Why are NIPS Tests Used in Prenatal Care?
NIPS tests were initially designed to detect Down syndrome and were quite successful (98-99%), but this accuracy does not extend to extremely rare conditions (such as DiGeorge syndrome and Prader-Willi syndrome), though that hasn’t stopped multiple manufacturers like Natera and Harmony from offering these additional screenings in an attempt to outsell one another in recent years.
All NIPS screenings are offered as laboratory developed tests (LDTs) and can be performed using a small blood sample from mom’s arm, which is less invasive than traditional diagnostic testing. The problem is NIPS tests only provide information about the risk of a possible genetic abnormality, not an actual diagnosis, but the language surrounding the results appears conclusive and absolute when advertised by lab companies as “reliable” and “highly accurate” and something that can give patients “total confidence.” This false sense of security in NIPS efficiency can lead patients to believe there is a genetic abnormality when in reality, there isn’t one.
“While genetic non-invasive prenatal screening tests are widely used today, these tests have not been reviewed by the FDA and may be making claims about their performance and use that are not based on sound science,” said Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health in an agency press release. “Without proper understanding of how these tests should be used, people may make inappropriate health care decisions regarding their pregnancy. We strongly urge patients to discuss the benefits and risks of these tests with a genetic counselor or other health care provider prior to making decisions based on the results of these tests.”
The unfortunate reality is that proper follow-up diagnostic testing doesn’t always happen. According to the Times, a 2014 study found that 6% of patients who screened positive for rare conditions obtained an abortion without confirming the initial result. Not only are these additional diagnostic tests more invasive (often requiring the drawing of amniotic fluid or a sample of placental tissue), but they come with a small chance of miscarriage, can cost thousands of dollars, and sometimes can’t be performed until the second trimester (or later), which exceeds the timeframe for a legal abortion in certain states.
What’s more, the report cites recent examples where women have terminated pregnancies after receiving confirmed test results, and later through follow-up testing revealed the fetus was actually healthy. These are real women who had to make critical health care decisions based on incomplete information they believed to be certain. All patients deserve to be fully informed of the risks and limitations with genetic testing from the very beginning to best understand and weigh their options.
What Should You Do if You’re Pregnant?
If you’re currently pregnant, you’ve probably received brochures on available genetic testing from your provider. (The American College of Obstetricians and Gynecologists encourages testing for all pregnant people, but you have the right to decline at any point.) Certain factors like your age, health history and family history may affect whether or not screening is recommended (and for what conditions), so always check with your doctor first and voice any concerns you have.
If you’ve already been screened and received results that are positive, know that additional testing is the only way to provide an accurate diagnosis. Results of NIPS tests alone should not be used to make decisions about your pregnancy.
“A positive screening test result means that the fetus has a higher risk of having a genetic abnormality compared with the average risk. It does not mean that the fetus definitively has a genetic abnormality, or a condition caused by a genetic abnormality,” notes the FDA.
If you’ve received a negative NIPS test, you should also know that it can not rule out the possibility that the fetus has a genetic abnormality, according to the FDA. Patients should have an open and ongoing conversation with their providers regarding all genetic testing, including NIPS tests. A full list of recommendations for patients and providers to safely communicate about genetic non-invasive prenatal screening tests and potential false results can be found here.








